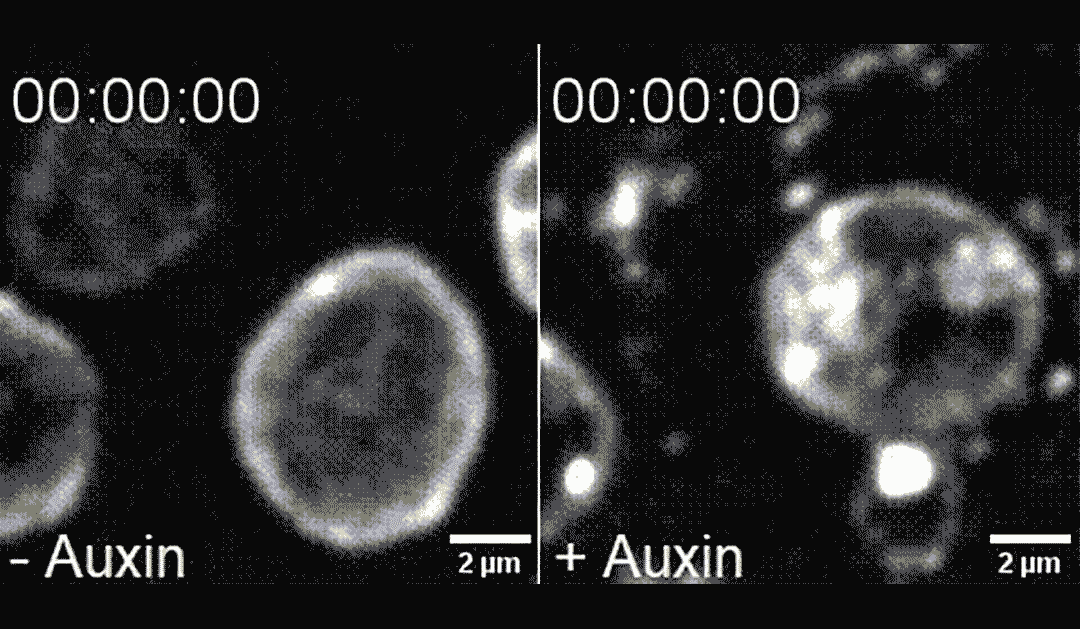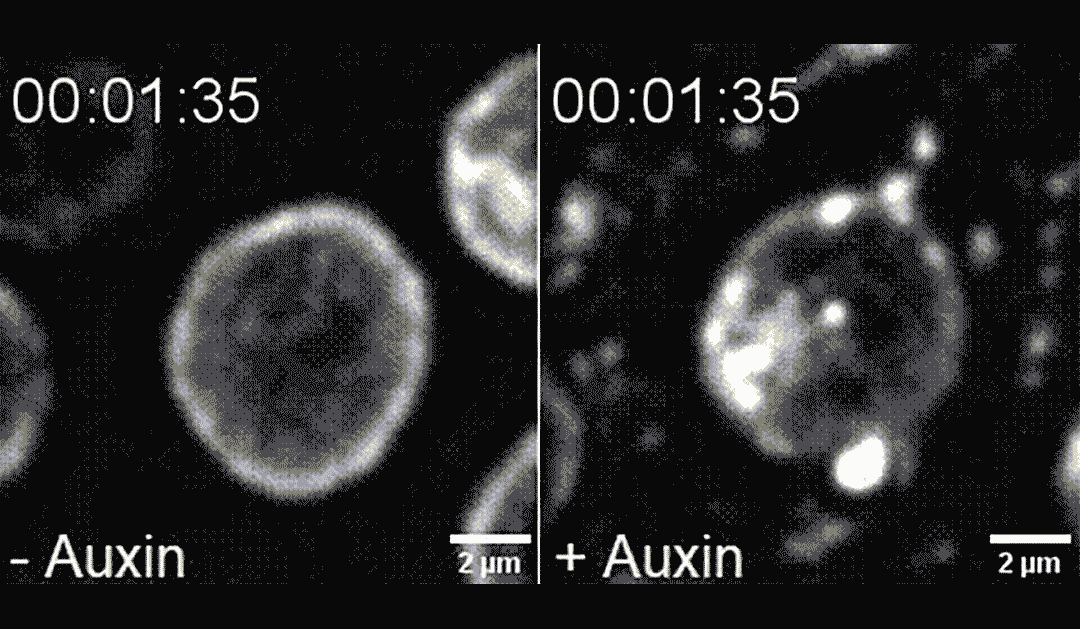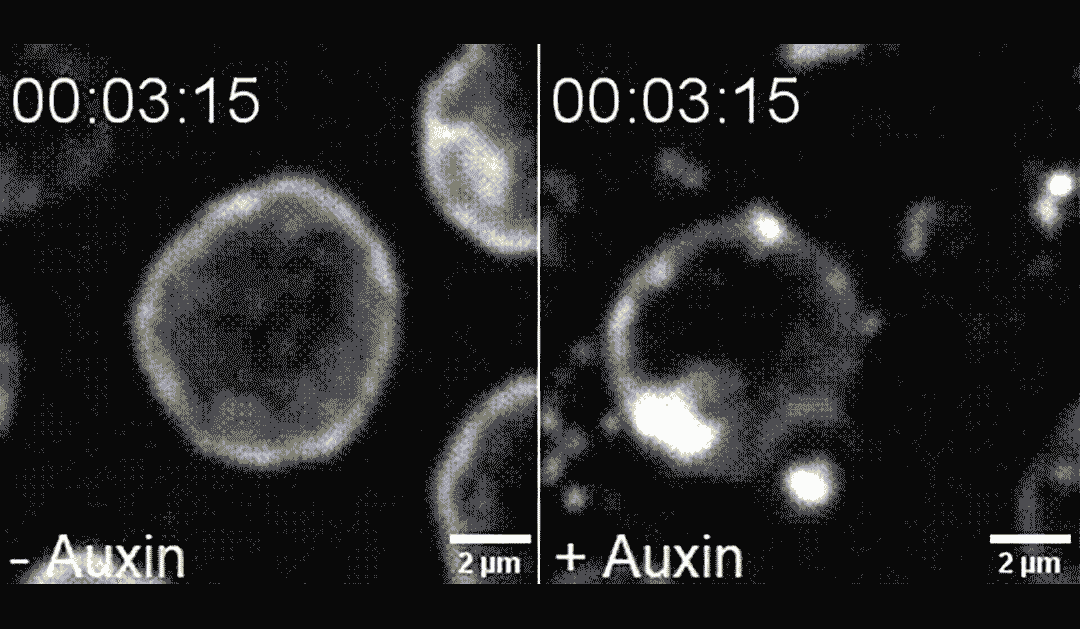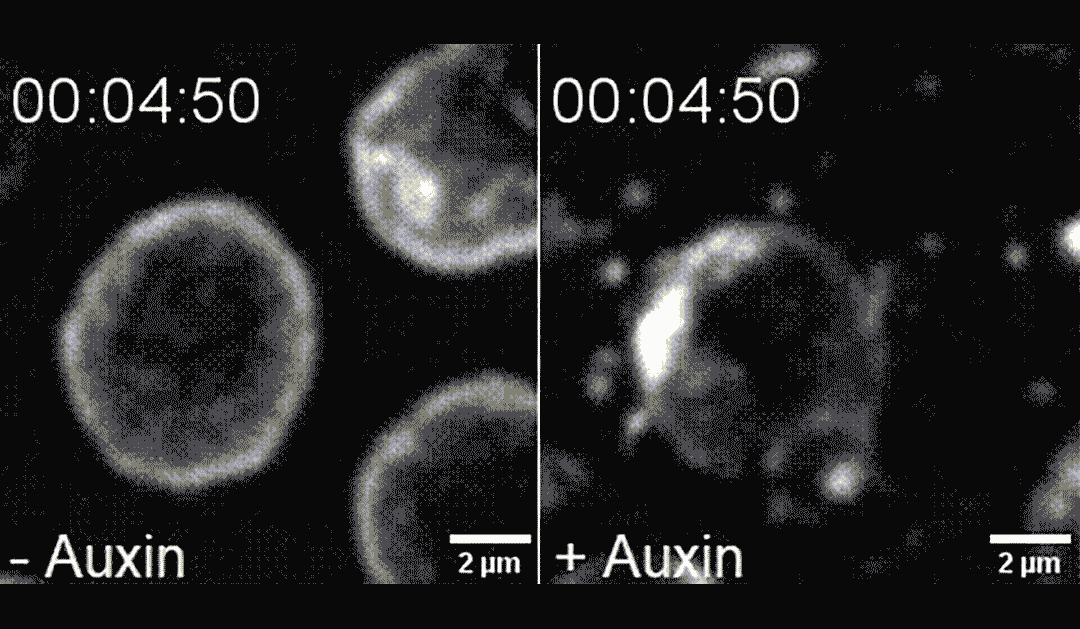
The researchers found that directing PLK-2 to chromosome ends at the nuclear envelope caused chemical modifications to the nuclear envelope, making it more pliable and less mechanically stable. This destabilization led to apoptosis, in a process dependent on the mechanosensitive ion channel called Piezo1/PEZO-1.
The connection to Piezo1 channels was unexpected. Piezo channels are better known for sensing mechanical forces at the cell’s outer membrane in tissues like skin and blood vessels, a discovery that was jointly awarded the 2021 Nobel Prize in Physiology or Medicine.
“This is the first time Piezo channels at the nuclear membrane have been linked to quality control during reproduction,” Liu said. “It shows that Piezo can also respond to events originating in the cell nucleus and this opens up a whole new area of research.”
Potential Links to Human Health
Although this research was conducted in tiny worms, similar quality control mechanisms could operate in mammals, including humans. Because errors associated with meiotic chromosome separation are a major cause of age-related decline in egg quality, this research on meiotic quality control could potentially benefit human reproductive health.
In addition, the CIP system developed in this study is a powerful tool that scientists can use to manipulate protein dynamics across various biological systems, with the potential to make significant impacts far beyond the field of reproductive health.
“Oocytes have a finite lifespan,” Liu said. “Those waking up later in life may have waited dormant for as long as 50 years! We hope this research provides new perspectives in understanding age-related oocyte quality decline, and one day can hopefully help extend the quality lifespan of these amazing, life-bearing cells.”
Story by Dan Armstrong