“If we could find a condition either using a genetic manipulation or some type of tool where we can figure out how that spreading is happening mechanistically and then find a way to stop it, could we potentially identify some potential therapeutic target or drug target?” he asks. That, he says, would allow researchers who design drugs to treat patients “to have something they could try and aim for, instead of just designing compounds in the dark.”
Babcock notes that with people living longer, the potential of more and more people developing neurodegenerative diseases looms larger.
“This is not only a fascinating area of research,” he says of his team’s work, “but something where we can hopefully make a real difference that will matter to people.”
The Hows of Fruit Fly Research
by: Stephen Gross
The objective is clear: Daniel Babcock's students typically set out to model different types of neurodegenerative diseases in fruit flies. But how exactly do students go from creating the flies they want to study to actually getting them under the microscope?
Knowing the age of the flies is crucial, so the first step is seeding a new vial. Researchers use CO2 to anesthetize several flies from the breeding stock, place them on a pad, and add to a new vial a number of males and females.
Once embryos develop, it takes about 10 days for adult flies to emerge, which are then collected and momentarily anesthetized with CO2 and sorted by gender.
"They can get a little bit scrappy when you have males and females together," Babcock says. "They will fight over resources, they will fight over mates."
The flies are then aged, and researchers change the vials every few days to keep their food fresh.
Once the flies reach 20 or 30 days old, some will begin to show symptoms of a disease mutation, like Parkinson's. Other "wild-type" flies are used as a control. Researchers then dissect the fly brains they want to examine.
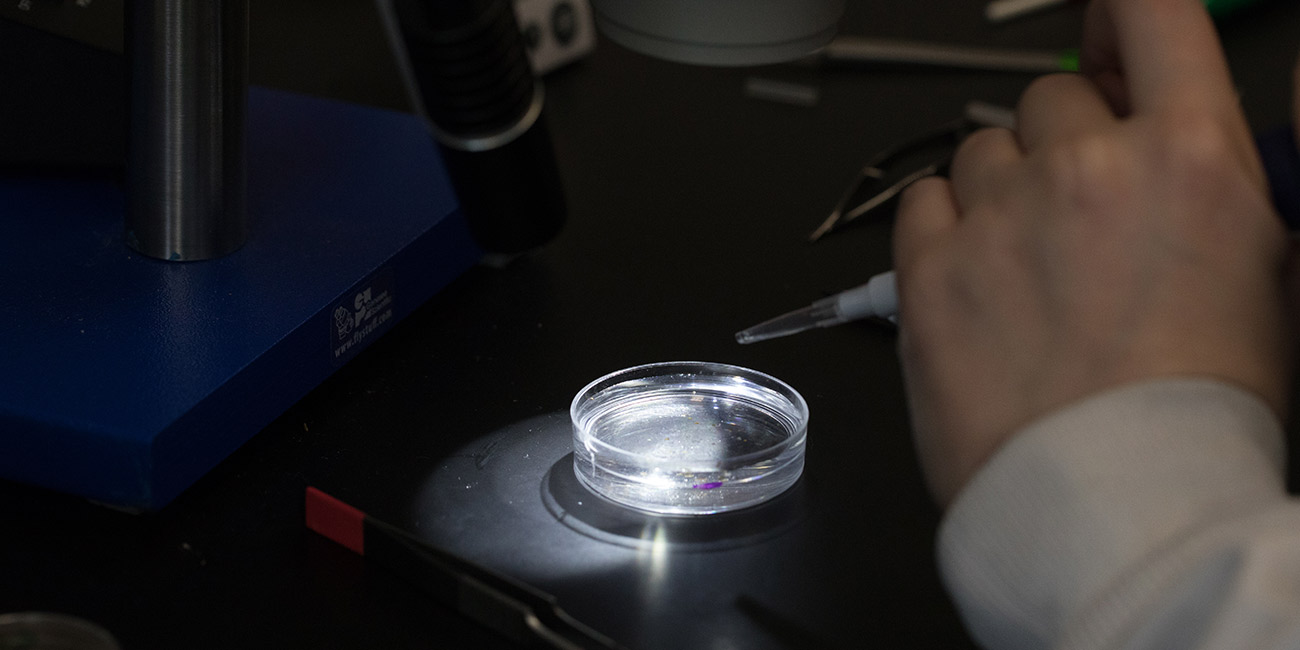
The selected flies are once again anesthetized with CO2 and transferred to a petri dish with a saline solution called PBS. The solution keeps the tissues from deteriorating and maintains a stable environment until the brains can be preserved. Brain dissection, through which 10 to 20 brain samples per condition are collected, occurs under a microscope.
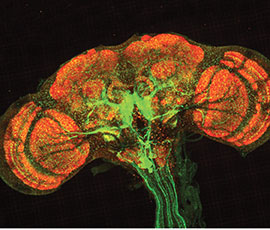
Before a researcher can examine the brain, they must preserve it with formaldehyde. Once preserved, the brain can be labeled with different antibodies. These antibodies can then be tagged with fluorophores of different colors, allowing particular proteins to light up within the brain.
Finally, researchers wash off the brain samples, place them on a microscope slide and cap them with a coverslip. Then, fluorescent images can be taken under the microscope.
Daniel Babcock earned his Ph.D. in neuroscience from the University of Texas Health Science Center at Houston/MD Anderson Cancer Center. He did his post-doctoral work at the University of Wisconsin- Madison. The Babcock Lab at Lehigh is interested in understanding the cellular and molecular mechanisms underlying the earliest hallmarks of neurodegenerative diseases.