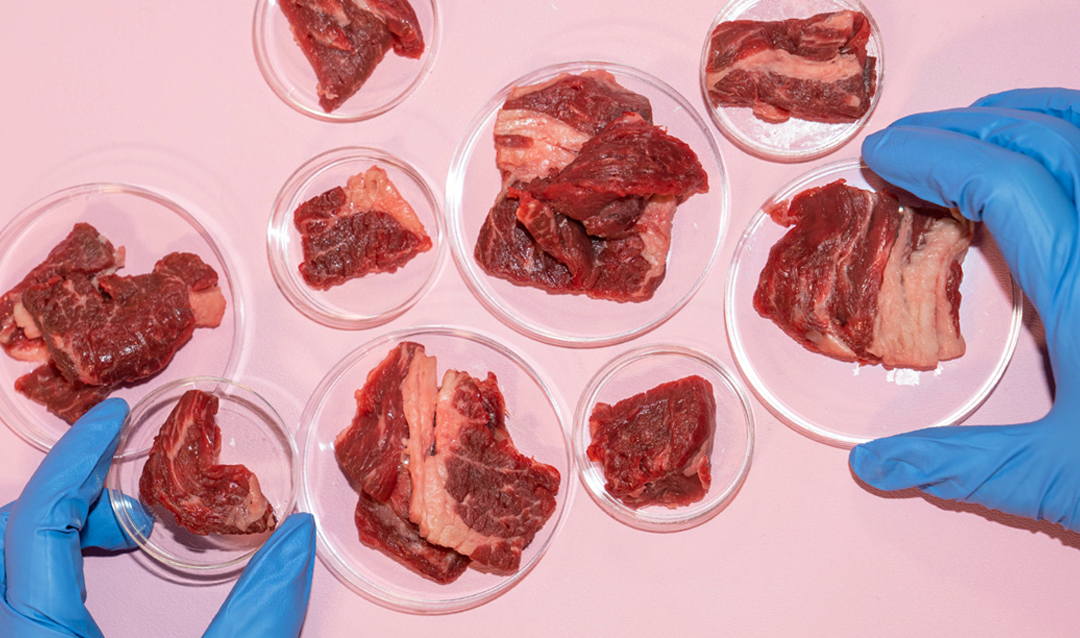
Schultz knows a lot about tissue engineering. Her expertise in the area of rheology—the study of the flow of fluids—contributes to the creation of hydrogel materials for biological applications such as wound healing, drug delivery and tissue regeneration.
Schultz says the leap from generating human tissue to animal tissue is not a big one.
“The idea for growing meat was inspired by this and just uses a different cell. At the moment in humans, we have been mimicking adipose tissue, which is not structured or exercised and will be implanted so it does not need the oxygen and nutrients. It was a jumping-off point, but the meat project has many more dimensions due to the highly collaborative nature of it.”
In 2021, Schultz received a $250,000 research grant from the Good Food Institute, a nonprofit think tank and international network of organizations working to accelerate alternative protein innovation. The two-year grant is seed money, says Schultz, who hopes the research continues beyond that.
Why Cultivated Meat is Important
Meat is a good source of energy and essential nutrients, including protein and iron, zinc and vitamin B12. The world is full of meat lovers—the global per capita consumption of meat continues to rise, more so in affluent countries.
But meat production is extremely damaging to the environment in many ways. By some estimates, meat production already requires more than half of the world’s estimated agricultural capacity. Meat production is responsible for 57 percent of global greenhouse gas emissions, leading to climate change, according to a study published in Nature Food.
A quarter of the planet’s ice-free land is used to graze animals used for meat, and a third of all cropland is used to grow food for them, according to the U.N. Food and Agricultural Organization.
Converting land to agricultural fields leads to species extinction because of the destruction of natural habitat, experts agree. Growing feed requires vast amounts of water and results in chemical contamination of land. In addition, manure decomposition releases harmful emissions, including methane, ammonia and carbon dioxide.
The hope is that lab-grown meat production would be more energy-efficient and result in lower gas emissions. The U.N. Intergovernmental Panel on Climate Change has said cultivated meat would be “transformative” in mitigating emissions.
Asked why the answer to the problem isn’t “just stop eating meat,” Schultz says the consumption of meat is too culturally ingrained. People will always want to eat meat. And for those who might be socio-economically disadvantaged, meat is an important source of nutrition. Cell-grown meat also could be an alternative for those who don’t eat meat because they consider it cruel to animals.
The Cultivated Meat Race
According to the Good Food Institute, more than 100 startups globally are focused on developing cultivated meat products while 64 companies have announced a business line in cultivated meat. Consultants McKinsey and Co. say the global market for cultivated meat could reach $25 billion by 2030.
Producers of lab-grown meat got a lot of attention at the 2022 United National Climate Change Conference, COP27. Mosa Meat CEO Maarten Bosch spoke on a panel with other alternative protein leaders, policymakers and investors. Good Meat held dining events to show off its newest version of cultivated chicken.