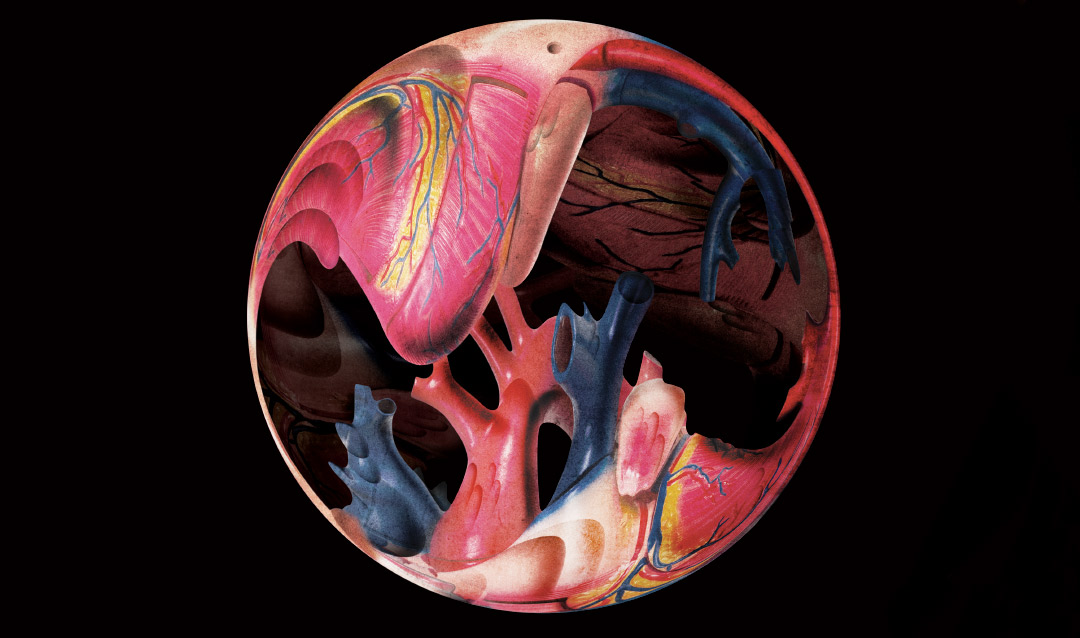
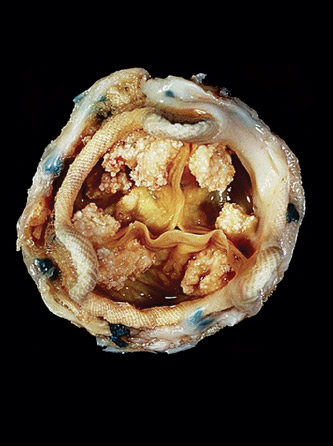
“Mathematically, it’s a very challenging problem,” says Yue Yu, an assistant professor of mathematics. “When damage [to the bioprosthetic heart valve] initiates, it’s very difficult to observe it. Even if you do an accelerated experiment, it still takes multiple years to open and close the heart valve [as many times as one would in 10 to 20 years]. It’s also very difficult to observe the change of the microstructure [of the valve] for every experiment, for each time you try to open and close the heart valve.”
Yu is developing a mathematical model and the corresponding open-source software to understand how this damage process evolves over time. She hopes this understanding can help researchers eventually improve the design—and extend the lifespan—of bioprosthetic heart valves.
“The problem for this [tissue] fatigue is even nowadays people still don’t understand what’s going on behind it, like how it gets developed and what is the underneath mechanism that causes it,” Yu explains.
With her mathematical model, Yu aims to capture the interactions between the tissue fibers and the underneath matrix of the bioprosthetic heart valve, and, in doing so, capture just how damage initiates.
Bioprosthetic heart valve damage is related not only to the valve itself, but also to the pattern of blood flow around it. With that in mind, Yu and her team have developed what they call the flow structure interaction framework, which captures the interaction between blood flow and soft tissue—in this case, the arterial walls and bioprosthetic heart valve. But, says Yu, the flow structure interaction alone cannot explain how damage occurs.
“We can only observe how maybe this region has a higher load applying on it, but what’s underneath that? Why the certain pattern of loads causes the damage is still unknown, especially because this damage doesn’t happen in one or two cycles—it develops over 10 or 15 years. ... It’s still important to understand what is the underneath mechanism in the microscale.”
Yu and her team are developing open-source software to validate that their mathematical model actually captures the phenomenon of bioprosthetic heart valve damage. Using numerical codes, the software will simulate the velocity of blood flow and the movement of the heart valve. The researchers can then compare their simulations with the images captured by experiments.
Yu envisions the software being applied to other problems or adapted into other applications. Other researchers, for example, might use it to capture the movements of a brain aneurysm, or materials scientists might modify and apply the software to simulate damage progression in other materials.
“The life of the bioprosthetic heart valve has been capped at 10 to 15 years for many years now,” says Yu. “This is a super complicated real-world problem: How can we try to translate that into the mathematical modeling and try to connect it with developed mathematical techniques? That interests me.”
Yu's research is supported by a CAREER Award from the National Science Foundation.
Illustration by Mario Hugo
This story originally appeared as "Modeling the Heart" in the 2019 Lehigh Research Review.